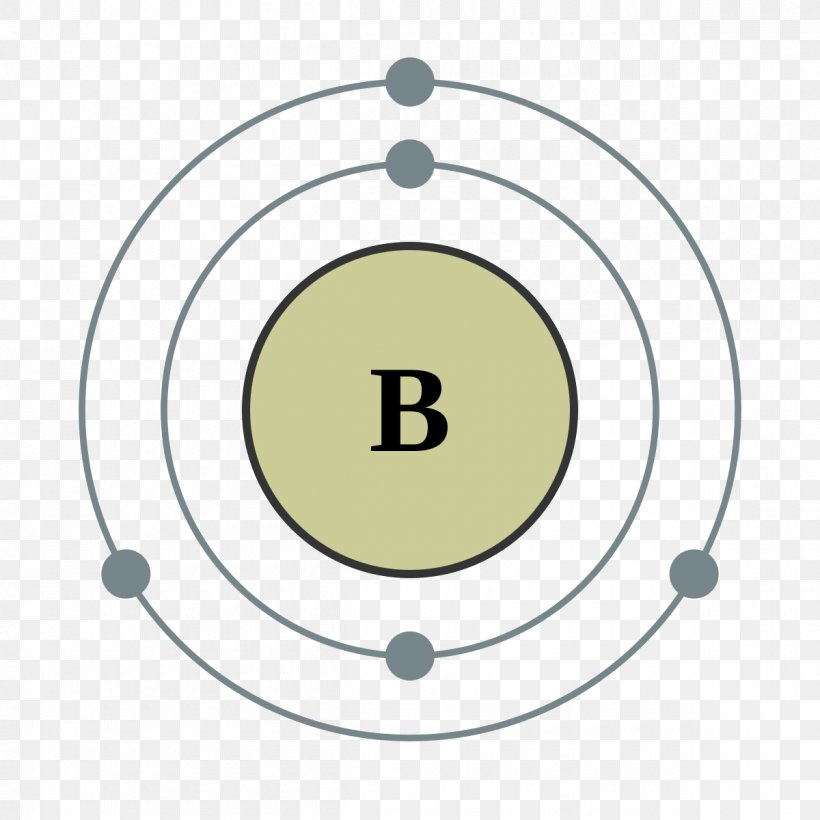
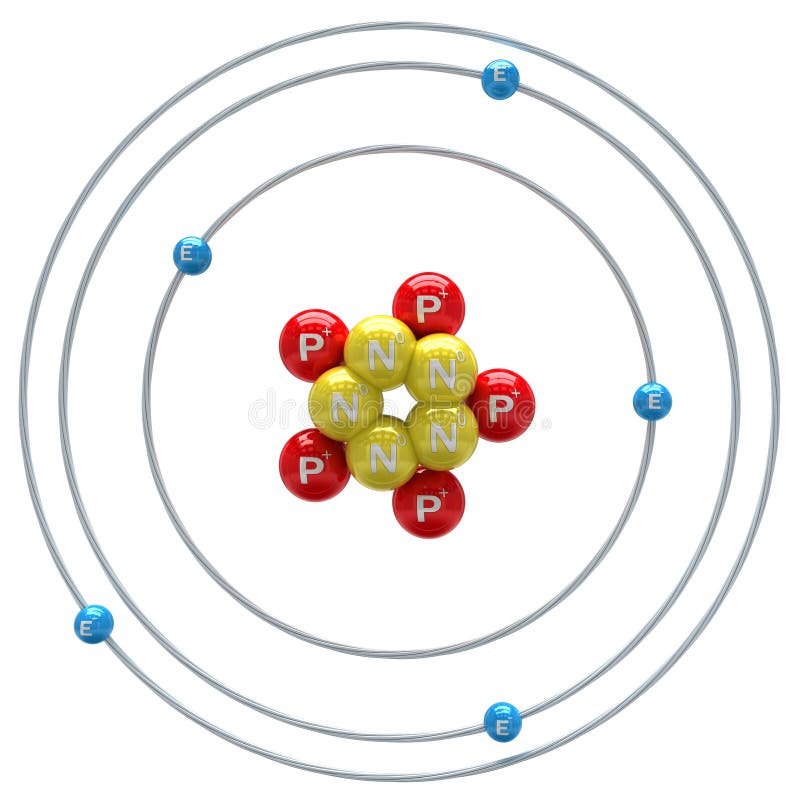
Boron Atom Model
Here's how you can draw the orbital diagram of boron step by step. #1 Find electrons of boron. #2 Write electron configuration of boron. #3 Draw orbital diagram of boron. Let's break down each step in detail.

Atomic Structure (Bohr Model) for Boron (B) YouTube
The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another. Only certain electron orbits are permitted.
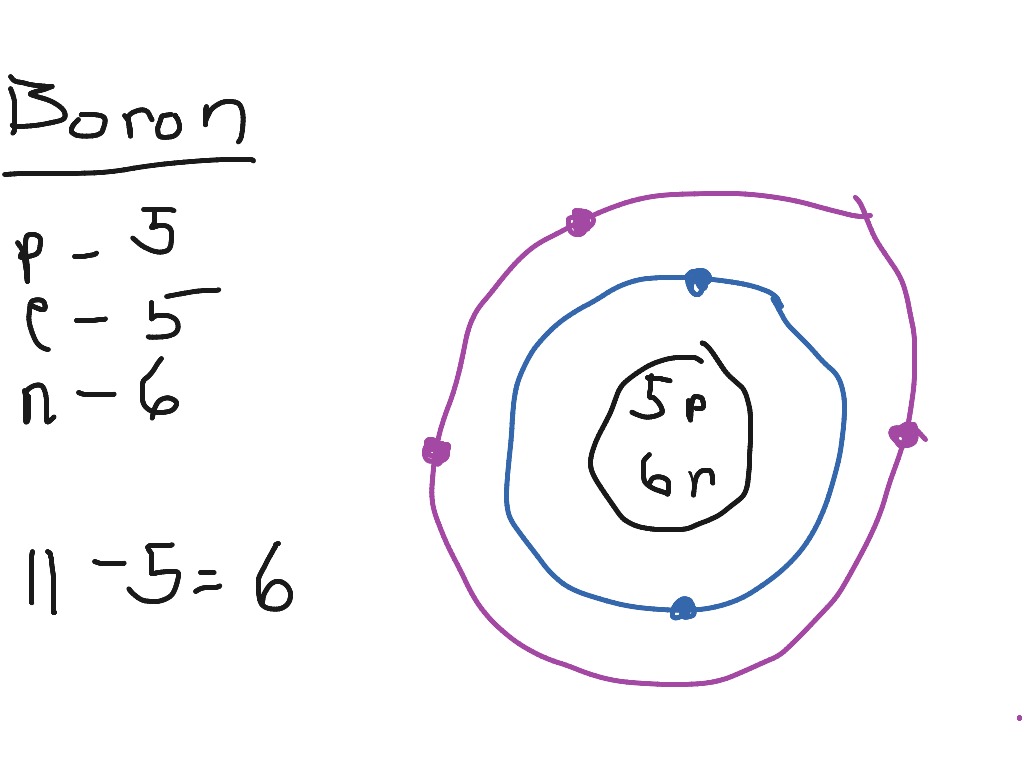
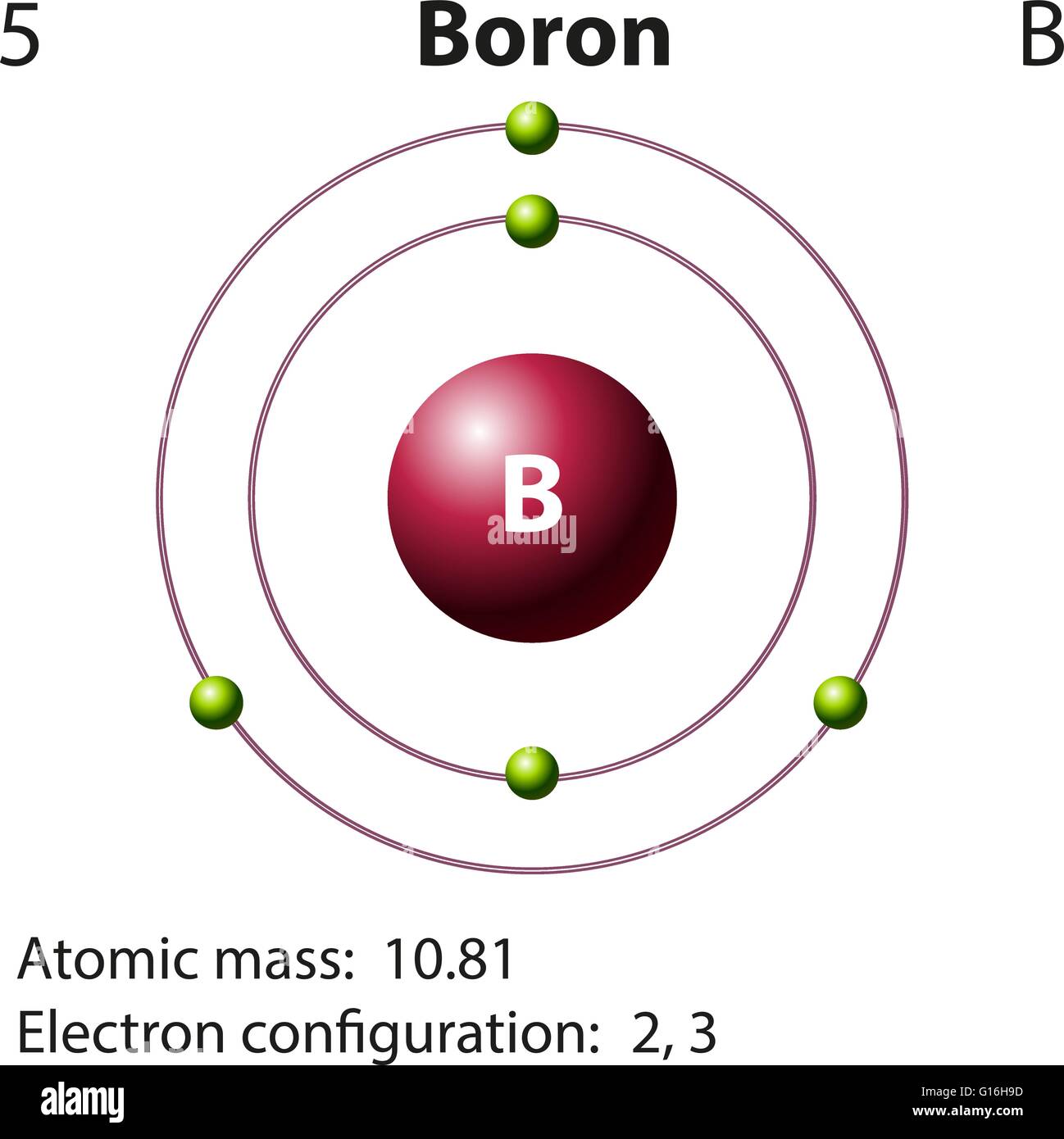
Boron Bohr Diagram
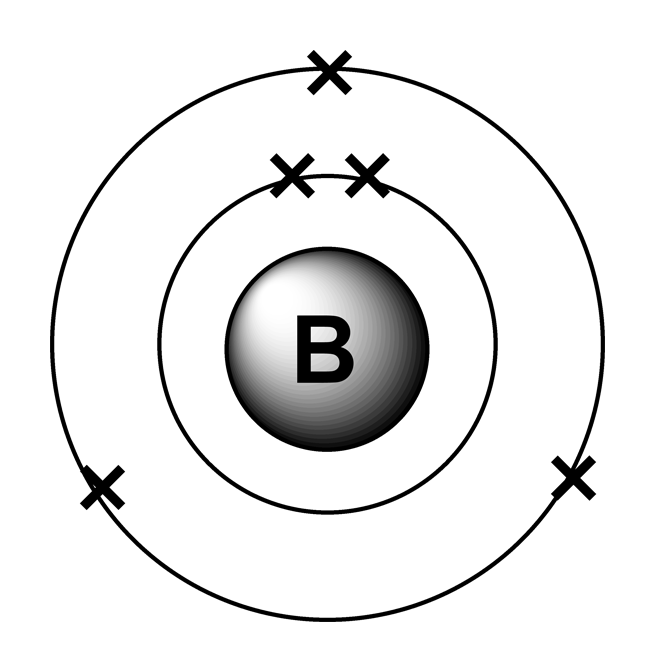
Boron has 2 electrons in its first shell and 3 in its second shell.Check me out: http://www.chemistnate.com
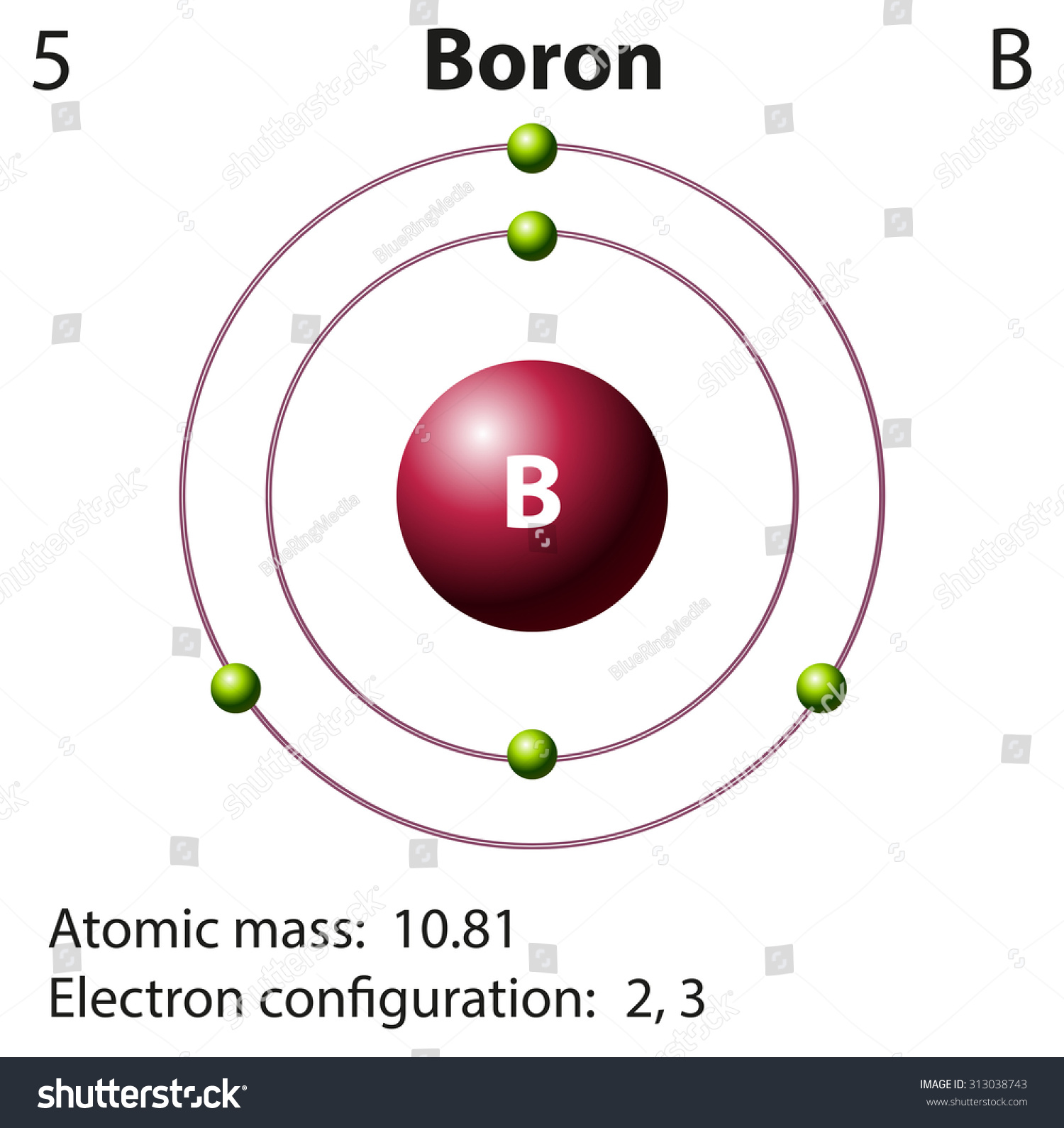
Diagram Representation Element Boron Illustration Stock Vector
Manish Bhardwaj. 5.6: Bohr Diagrams of Atoms and Ions is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells,..
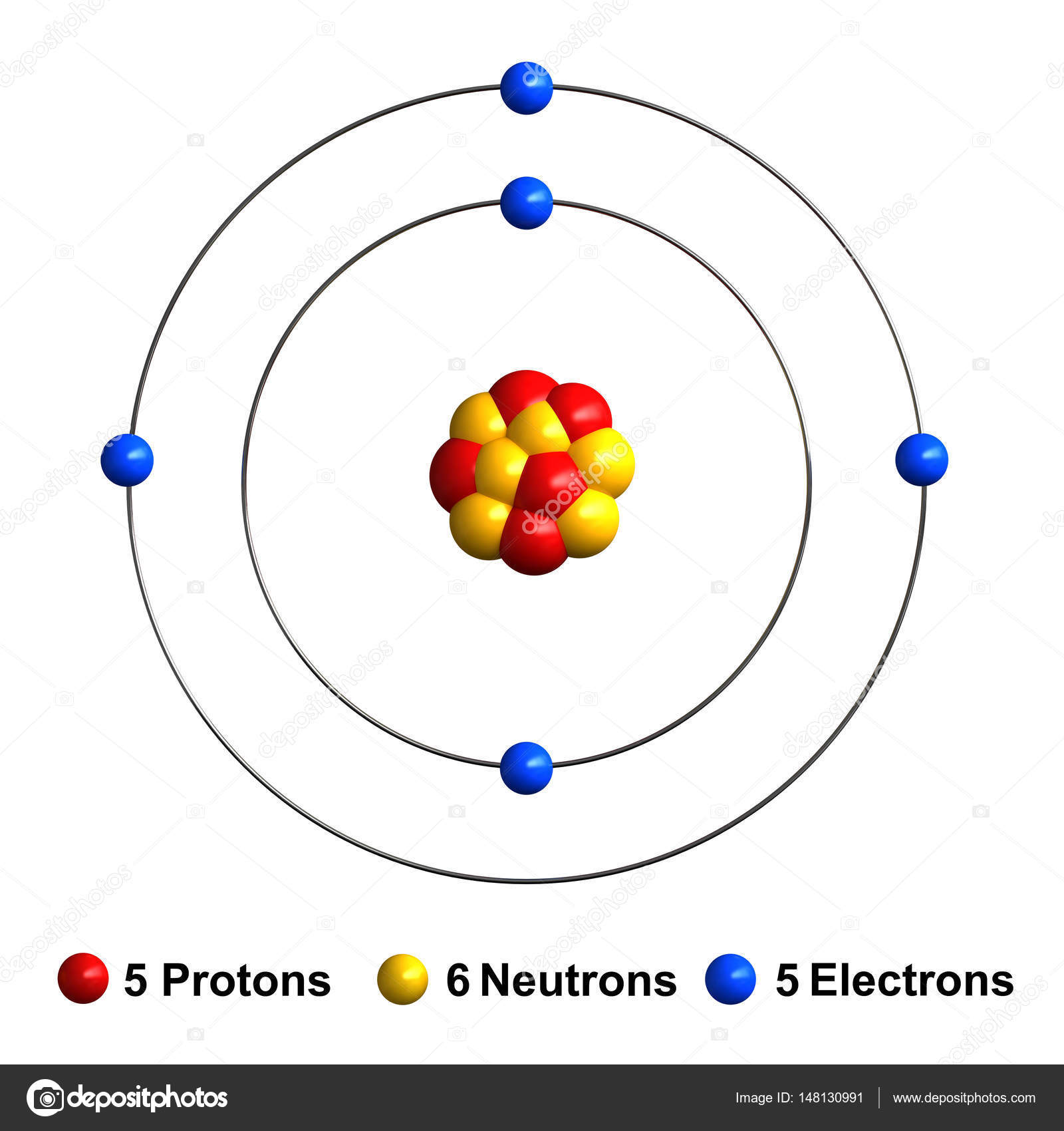
3d render of atom structure of boron Stock Photo by ©oorka5 148130991
What is the Bohr diagram for boron? Diagram of an Atom: A Bohr diagram can be used to visually show the Bohr model of a particular atom. An atom is the smallest building block of an element and each element has a specific number of subatomic particles known as protons, neutrons and electrons. Neils Bohr, a Danish scientist, made the discovery.

Boron Bohr Diagram
Steps Write protons, neutrons, and electrons of boron atom Boron has 5 protons, 6 neutrons, and 5 electrons. Learn how to find: Boron protons neutrons electrons Draw nucleus of boron atom The nucleus of a boron atom contains 5 protons and 5 neutrons. So draw the nucleus of boron atom as follows: Boron nucleus
Boron stock illustration. Illustration of symbol, electrons 89682672
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have. Figure 2 2 contrast the Bohr diagrams for lithium, fluorine and aluminum atoms. The shell closest to the nucleus is.

Boron Bohr Model Chemistry Pinterest Bohr model, Chemistry and
Course: Class 9 Chemistry (India) > Unit 4. Lesson 1: Models of an atom. Discovery of the electron and nucleus. Rutherford's gold foil experiment. Drawback of the Rutherford model. Bohr's model of an atom. Atomic structure. Science >. Class 9 Chemistry (India) >.

Bohr Structure Boron Atomic Physics Stock Vector (Royalty Free
Bohr Diagram: Lewis structure: B 5 B Boron 10.81 Step 5: Use the following colors to shade in the square for each element. You should ONLY color in the small square in the upper left-hand corner and not the entire card. Green = Li & Na Orange = B & Al Pink = O & S Red = C & Si Blue = Be & Mg Tan = N & P Purple = F & Cl Yellow = He, Ne, & Ar
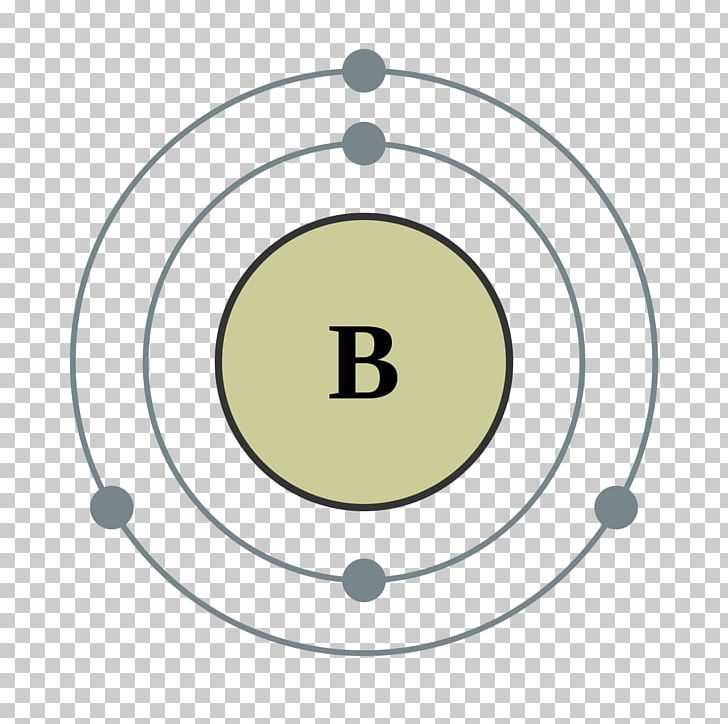
33 Boron Shell Diagram Wiring Diagram Info
In this article, we'll study the Boron Bohr Model and will go through the steps to draw its Bohr Diagram. The boron Bohr model provided new information on the atom and its structure. Contents show Boron Bohr Model The Bohr Model or precisely Rutherford-Bohr model was presented in 1913 by Niel Bohr and Ernest Rutherford.
Boron Electron Dot Diagram Photos
In this video we'll look at the atomic structure and Bohr model for the Boron atom (B). We'll use a Bohr diagram to visually represent where the electrons ar.
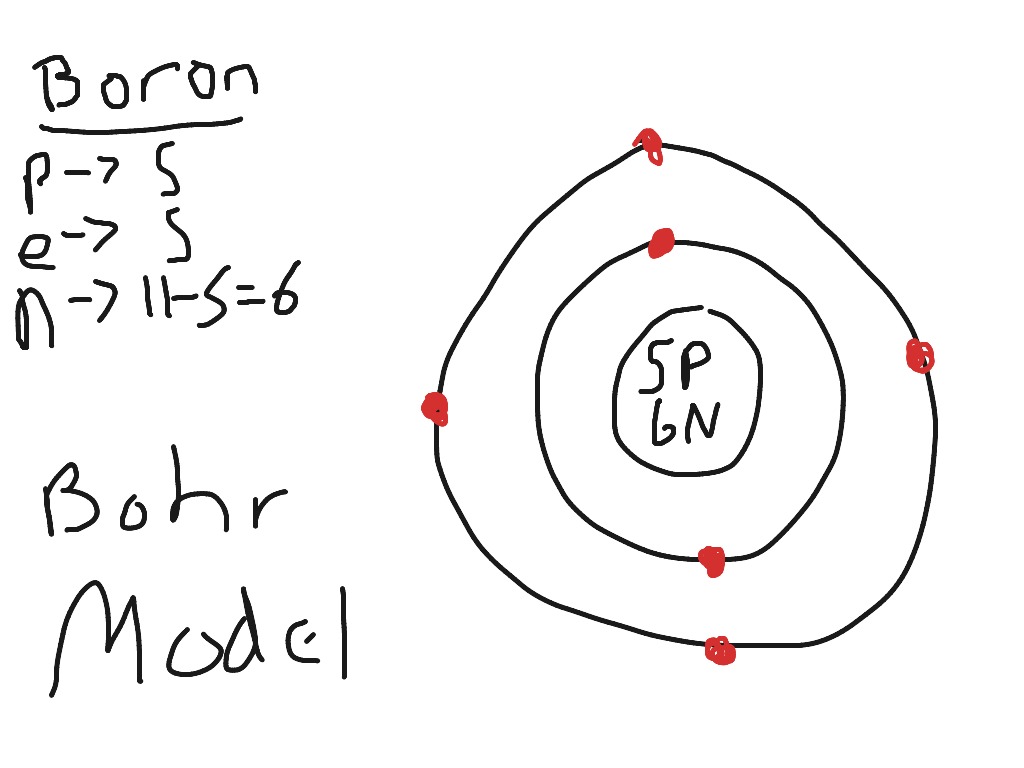
Boron Bohr Diagram
Bohr model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr.The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models. The Bohr model and all of its successors describe the properties of.

Boron, atomic structure Stock Image C018/3686 Science Photo Library
Bohr's model calculated the following energies for an electron in the shell, n. . : E ( n) = − 1 n 2 ⋅ 13.6 eV. Bohr explained the hydrogen spectrum in terms of electrons absorbing and emitting photons to change energy levels, where the photon energy is. h ν = Δ E = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 eV.

See the Electron Configuration of Atoms of the Elements
What is Boron. Boron (pronunciation BO-ron [2]), represented by the chemical symbol or chemical formula B [1], is hard and brittle in its crystalline form [22].It has allotropes in the form of an amorphous powder and three major crystalline forms [34].Naturally occurring B has two stable isotopes with mass numbers 10 and 11 [1, 2].Besides that, it has 11 synthetic isotopes, some of which are.
Electron arrangements
This page titled 4.2: The Bohr Model is shared under a CC BY license and was authored, remixed, and/or curated by OpenStax. Bohr incorporated Planck's and Einstein's quantization ideas into a model of the hydrogen atom that resolved the paradox of atom stability and discrete spectra. The Bohr model of the..

Boron Number Of Electrons
Bohr Model of all Elements (Diagrams + Chart) March 23, 2023 by Jay Bohr model of all Elements is mentioned in the chart below. Free Gift for you: Interactive Periodic Table Let me tell you how this Interactive Periodic Table will help you in your studies. 1).