PPT Introduction to Solutions PowerPoint Presentation, free download
For example, if you prepare a saturated solution of sugar in hot water and then cool the solution, it becomes supersaturated when the temperature changes. Disturbing the solution or adding a nucleation point (like a seed crystal or even a scratch on the container) induces crystal growth. Examples of Saturated Solutions

Saturated Solution Easy Science Easy science, Chemistry experiments
Examples of Saturated Solution: 1. Drinking Beverages. One of the most widely seen and possibly widely enjoyed saturated solutions is a carbonated beverage, like soda. The solution, in this case the water that forms the base of the soda, is bombarded with carbon until no more can be introduced, meaning it gives off the excess carbon as gas.

PPT Topic 7 Solutions PowerPoint Presentation, free download ID5738045
Examples of saturated solution in a sentence, how to use it. 26 examples: The uranium solution was a twofold dilution of a saturated solution. - The…

What is Saturated Solution AmirecMccullough
What is a supersaturated solution? A supersaturated solution contains more solute at a given temperature than is needed to form a saturated solution. Increased temperature usually increases the solubility of solids in liquids. For example, the solubility of glucose at 25 °C is 91 g/100 mL of water. The solubility at 50 °C is 244 g/100 mL of.
Saturated Solution Class 6, Separation of Substances
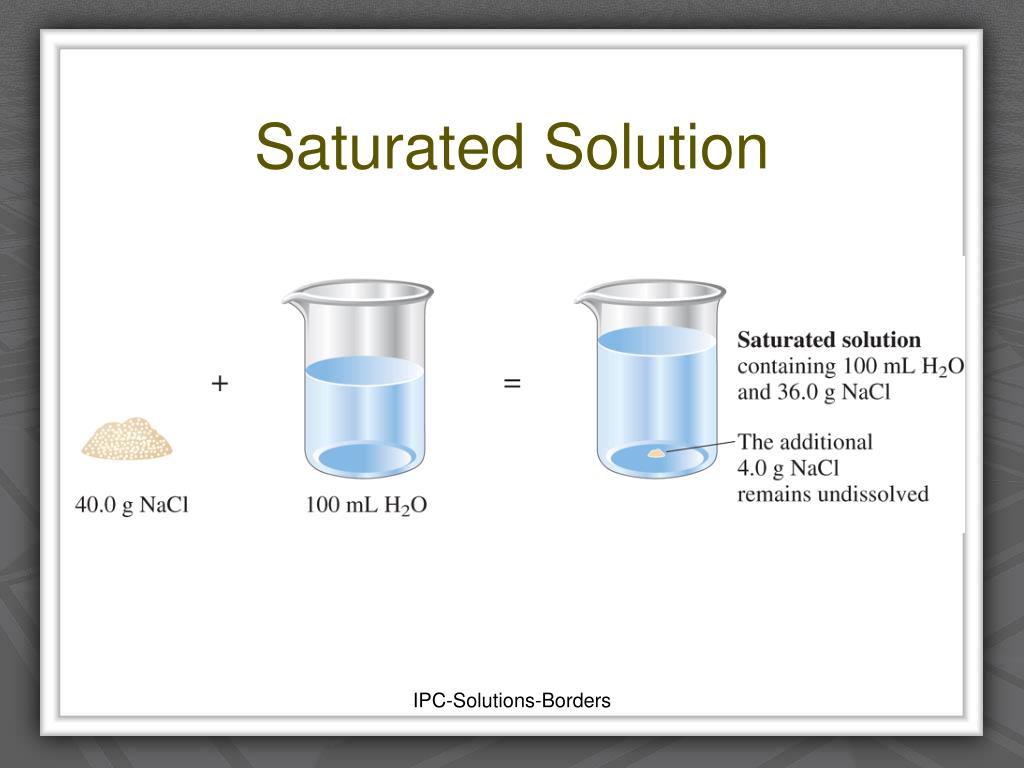
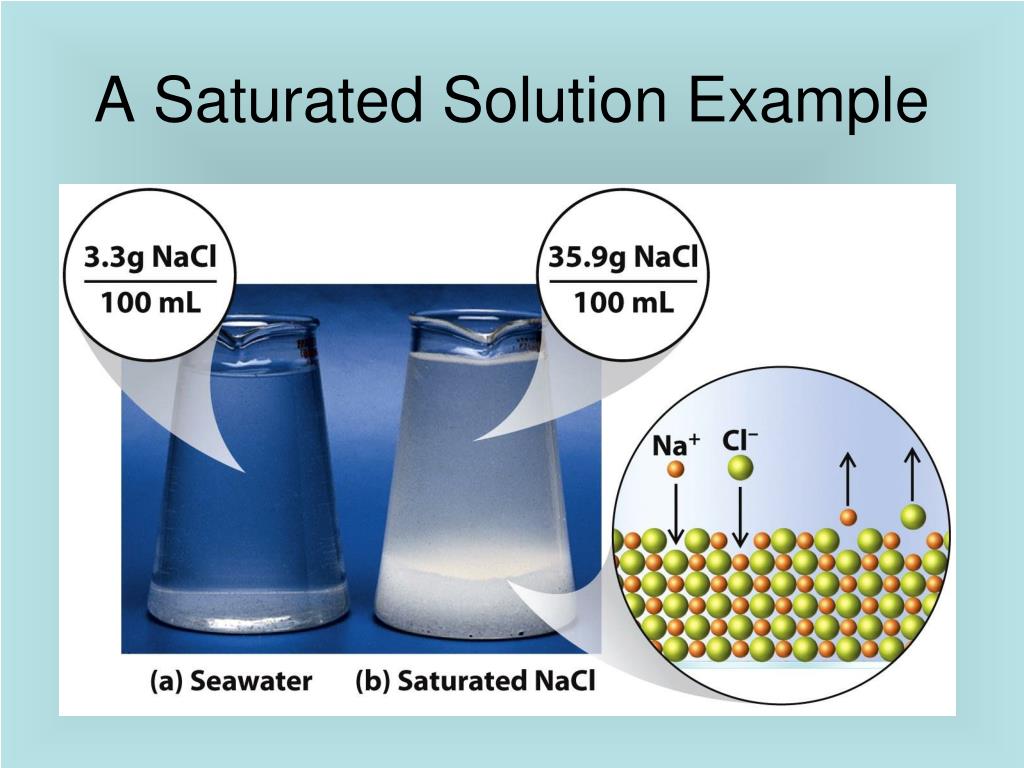
Using the value just stated, a saturated aqueous solution of NaCl, for example, contains 35.9 g of NaCl per 100 mL of water at 20°C. We can prepare a homogeneous saturated solution by adding excess solute (in this case, greater than 35.9 g of NaCl) to the solvent (water), stirring until the maximum possible amount of solute has dissolved, and.

Examples of Saturated Solution YourDictionary
1. Figure 7.10.1 7.10. 1 : An unsaturated solution and an exactly-saturated solution, respectively. These solutions can, however, be differentiated through the addition of more solute. Because an unsaturated solution does not contain the maximum of amount of solute that can dissolve in the quantity of solvent that is present, additional solute.

Supersaturated Solution Definition and Examples
Here are some common examples: A soda is a saturated solution of carbon dioxide in water. This is why, when the pressure is released, carbon dioxide gas forms bubbles. Adding chocolate powder to milk so that it stops dissolving forms a saturated solution.

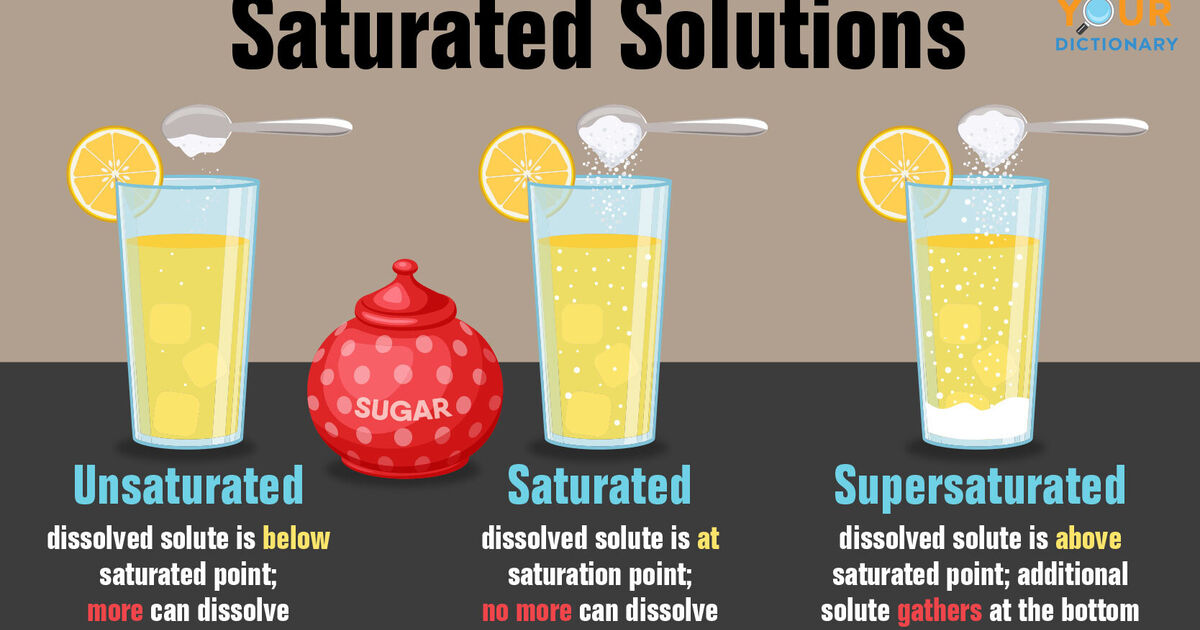
saturated unsaturated and supersaturated solutions Google Search
An unsaturated solution is a solution that contains less than the maximum amount of solute that is capable of being dissolved. The figure below illustrates the above process and shows the distinction between unsaturated and saturated. Figure 16.3.1 16.3. 1: When 30.0g 30.0 g of NaCl NaCl is added to 100mL 100 mL, it all dissolves, forming an.

PPT Introduction to Solutions PowerPoint Presentation, free download
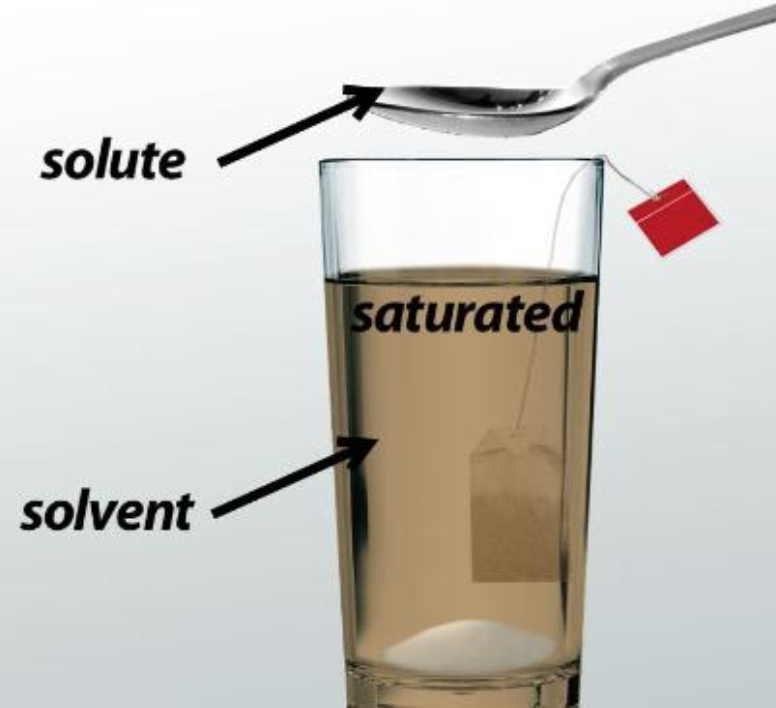
A saturated solution is one containing as much solute —the solid being dissolved in the liquid—as possible without forming a precipitate, or leftover solid. This is the maximum concentration of solute. How to Make a Saturated Solution Here are three ways to make a saturated solution : Add solute to a liquid until no more will dissolve.

Saturated Solution Definition & Examples Video & Lesson Transcript
These terms are also qualitative terms because each solute has its own solubility. A solution of 0.00019 g of AgCl per 100 g of H 2 O may be saturated, but with so little solute dissolved, it is also rather dilute. A solution of 36.1 g of NaCl in 100 g of H 2 O is also saturated, but rather concentrated. In some circumstances, it is possible to.

Saturated and Unsaturated Solutions Definition & Difference
In the first example, the solution is saturated when the rate at which the pure substance dissolves in the solvent to enter the solution is exactly equal to the rate at which the dissolved substance leaves the solution (e.g., by crystallizing). In the second example, the rate at which the pure condensed (liquid or solid) material vaporizes is.
PPT Chapter 4 PowerPoint Presentation, free download ID4638445
Examples of Saturated Solutions In everyday life, you find saturated alternatives, not just in a chemistry classroom. Also, water does not have to be the solvent. Here are some popular instances: Saturated carbon dioxide solution in water is a soda. That's why bubbles form when we remove pressure from carbon dioxide gas.

Saturated Solution Definition in Chemistry
Saturated Solution: It is a solution where the maximum amount of solute is, so much so that if there was any more, it would not dissolve. Unsaturated Solution: It is a solution where the solute concentration is lesser than its corresponding equilibrium solubility.

Difference between Saturated and Unsaturated Solution Teachoo
Some common examples of saturated solutions are : Saturated salt solutions, which is identified by the settling down of the salt in the bottom of the solution. Carbonated drinks are nothing but the saturated solution of carbon dioxide dissolved in the water. Get Unlimited Access to Test Series for 820+ Exams and much more.
Examples of Saturated Solution YourDictionary
Everyday Examples of Saturated Solution Beverages are one of the most widely used and loved saturated solutions. In these drinks, water is a solvent and carbon is bombarded as a solute until the point of saturation is reached. In the kitchen, many cooking recipes involves dissolving of salt, sugar and other household ingredients into the water.

What is Saturated Solution
A typical saturated solution example is air saturated with water vapor. When air, a solution of mostly nitrogen and oxygen, is saturated with water vapor (i.e., the concentration of water.